Fischer - Tropsch Process
Fischer-Tropsch (FT) process is the process where synthesis gas (H2 and CO) is converted into a mixture of hydrocarbons, oxygenates, water and carbon dioxide. The essential step, known as the Fischer-Tropsch (FT) reaction, can be written as
nCO + (2n+1)H2 → CnH(2n+2) + nH2O
where CnH(2n+2) represents a range of hydrocarbons, ranging from low-molecular-weight gases (n=1, methane), by way of gasoline (n=5-12), diesel fuel (n=13-17), and as far as solid waxes (n>17). The FT reaction is usually a catalytic reaction at high temperatures (180-350oC) and high pressure for realistic rates to be achieved, and the typical catalysts used are based on iron or cobalt. The hydrocarbons produced by FT process can be refined and used in place of more conventional liquid fuels derived from crude oil. Synthetic fuel can be produced by a variety of gasification methods, with gas-to-liquid (GTL), coal-to-liquid (CTL) and biomass-to-liquid (BTL) being the most widespread.
Process Flow Diagram

(Source: Speight, JG, Synthetic fuels handbook: properties, process, and performance, McGraw-Hill, 2008)
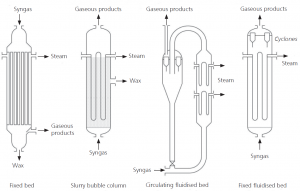
Equipment
(Source: Mousdale, DM, Biofuels: biotechnology, chemistry, and sustainable development, CRC Press, 2008)
Process Videos
Gas to Liquids Process
Biomass to Liquid Process
Coal to Liquid Process
Four Types of F-T Reactors
Glossary
References
- Speight, JG, Synthetic fuels handbook: properties, process, and performance, McGraw-Hill, 2008
- Mousdale, DM, Biofuels: biotechnology, chemistry, and sustainable development, CRC Press, 2008