Vacuum Pyrolysis
Vacuum pyrolysis takes advantage of a vacuum system within the actual reactor; this vacuum is basically a sub-atmospheric pressure within the reactor. Because of this type of system, the boiling point of substances within the reactor will be less than what they would at atmospheric pressure; carbon conversion can be more easily reached using this type of pyrolysis, and it can become easier to avoid side reactions from occurring. In flash vacuum pyrolysis, the temperature needed for conversion can be lowered quite substantially; a type of distillation process is used in which the fuel travels through a in which the lower pressure and high temperatures are implemented. Residence time is quite short in this type of chemical process because of the very low operating pressure; at the laboratory scale, the exposure time can be in the millisecond range.
Flow Diagrams
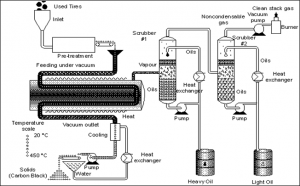
(Source: http://infohouse.p2ric.org/ref/11/10504/html/biblio/html4/pyh3.htm)
Equipment
Videos
Pyrolysis of plastic bags part 1.MOV
Thermal cleaning of candle filter sets in a SCHWING VacuClean System 654T
References
- http://en.wikipedia.org/wiki/Pyrolysis#Use_of_vacuum
- http://www.scripps.edu/baran/images/grpmtgpdf/Holte_Apr_12.pdf
- http://infohouse.p2ric.org/ref/11/10504/html/biblio/html4/pyh3.htm
- http://web.anl.gov/PCS/acsfuel/preprint%20archive/Files/31_3_ANAHEIM_09-86_0210.pdf
- http://www.youtube.com/watch?v=fguO9a-jECc
- http://www.youtube.com/watch?v=rbyUUt60XDQ