Liquefaction of Plastic Waste
The method of converting the polymers present in the waste plastics into liquid chemical products that can be used for various activities has shown dramatic potential results in the previous experiments conducted; as reported in an experiment conducted by James A. Guin and H. S. Joo of obtaining chemical product naphtha from waste polymer liquid through pyrolysis. Not only naphtha, but also reports of converting waste polymers into fuel has been also reported using methods such as thermal cracking, flow cracking, catalytic cracking, and pyrolysis. In thermal degradation process, waste polymers are heated at a temperature range of 370–420 °C to de-polymerize the polymers of plastics back to liquid form. The conversion process yields 80–90% liquid, 5% light gas and 5% residue materials. The thermal de-polymerization is carried out in a fractional distillation process, where other liquid grade fuels are also obtained based on temperature profiles used. The naphtha liquid is obtained when the temperature reaches 110 °C. The general term for thermal degradation is applying heat in order to breakdown long hydrocarbon chains to form shorter ones thus producing a new substance. In order to produce and obtain different categories of liquid products the thermal degradation process is upgraded to fractional distillation process. Fractional distillation process is basically thermal degradation except the heat applied is divided into different groups based on specific temperature. The long hydrocarbon chain lengths of plastics are broken down to form shorter hydrocarbon chain lengths of C3–C27. Further, during fractional distillation, these chains are divided into different groups based on the type of liquid produced. The naphtha liquid’s carbon chain is in the range of C6–C14. Through this process, other commercial grade fuel substances are also produced. Moreover, the process is very efficient; it exerts traces of light gas, although this gas can be utilized as a heat source.
Flow Diagrams
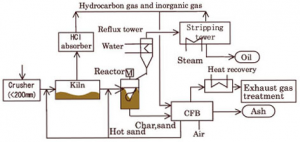
(Source: http://www.gec.jp/JSIM_DATA/WASTE/WASTE_2/img/Fig_403-1.jpg)
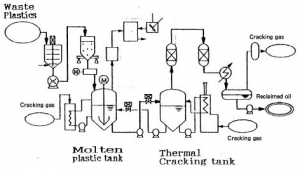
(Source: http://www.gec.jp/JSIM_DATA/WASTE/WASTE_2/img/Fig_402-1.jpg)
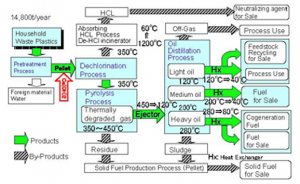
(Source: http://www.kleanindustries.com/i/photos/solutions/SRP_plastic_recycling_flow_chart.jpg)
Equipment
Videos
References
- http://www.ashdin.com/journals/JFREA/R110101.pdf
- http://www.gec.jp/JSIM_DATA/WASTE/WASTE_2/img/Fig_403-1.jpg
- http://www.gec.jp/JSIM_DATA/WASTE/WASTE_2/img/Fig_402-1.jpg
- http://www.kleanindustries.com/i/photos/solutions/SRP_plastic_recycling_flow_chart.jpg